Lumipulse® G SARS-CoV-2 Ag
For in vitro diagnostic (IVD) use with the Lumipulse G system for detection and quantitative measurement of SARS-CoV-2 nucleocapsid protein antigen in human nasopharyngeal swab or saliva. As a diagnostic tool for the confirmation of a SARS-CoV-2 infection.
The assay utilises proven CLEIA (chemiluminescent enzyme immunoassay) technology with results that are available in up to 35 minutes.
CE marked
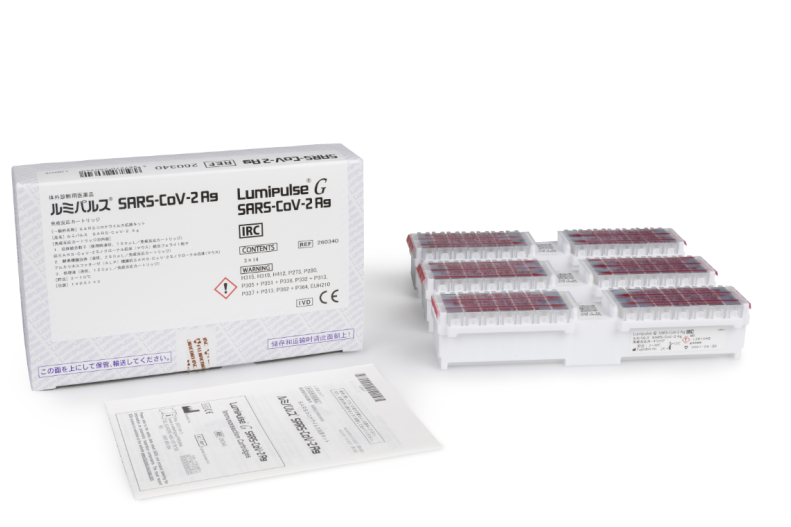
Lumipulse® G SARS-CoV-2 Ag Immunoreaction Cartridges
Product number 260340
3 x 14 Tests
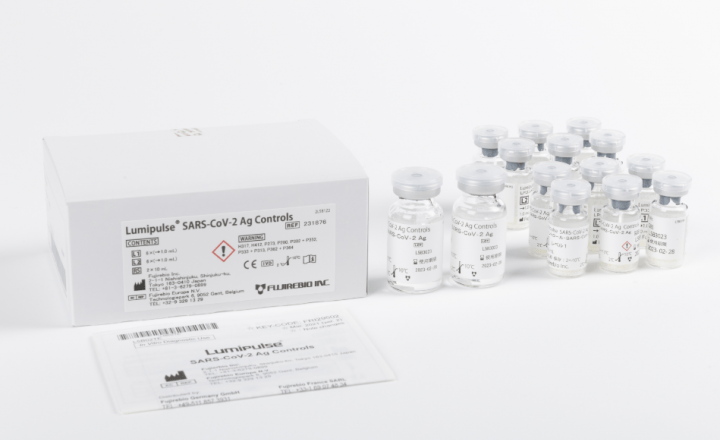
Lumipulse® G SARS-CoV-2 Ag Calibrators
Product number 231869
4 x 4 Concentrations
Lumipulse SARS-CoV-2 Ag Sample Extraction Solution set for Nasopharynx swab
Product number 231883
20 Tests
Sample Extraction Solution
Product number 234921
4 x 40mL
Sample Extraction Solution set
Product number 234914
50 Tests
Please contact your local Fujirebio representative for the availability of this product in your country.