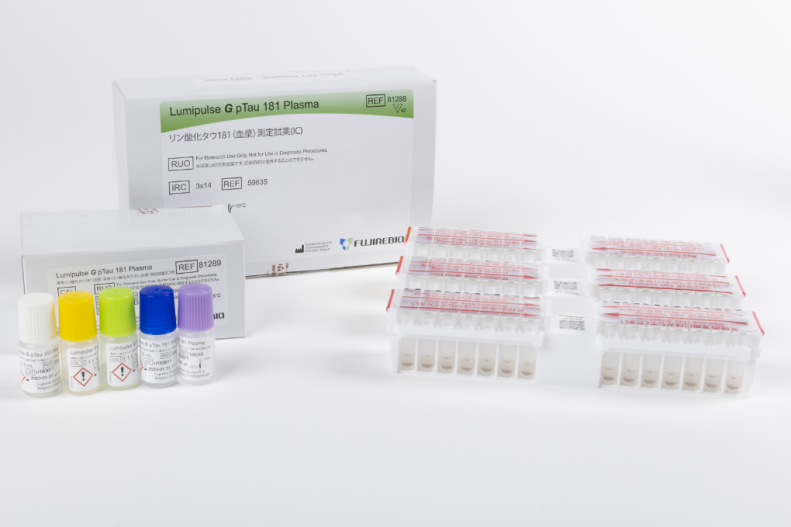
Lumipulse® G pTau 181 Plasma
Immunoassay to be used with the automated LUMIPULSE G System for the quantitative measurement of Tau phosphorylated at threonine 181 (pTau 181) in human plasma. This product is not for use in diagnostic procedures.
Product number 81288
Product number 81289
Click here to navigate
- Contact sales for information
- Details
- Conditions of sale
- Citations
- Documentation
- Insights
- Product inquiry
- Related products
- Webinars
-
Details
Background
Alzheimer's disease, the most common form of dimentia, is a neurodegenerative disorder histologically characterized by the accumulation of intracellular neurofibrillary tangles and extracellular amyloid plaques throughout the cortical and limbic brain regions. The ultrastructure of neurofibrillary tangles is made up of paired helical filaments composed mainly of abnormally hyperphosphorylated Tau protein (pTau). The major components of the amyloid deposits are the 40- and 42-amino acid-long β-amyloid peptides, which are derived from integral membrane-bound amyloid precursor protein.1
In Alzheimer's disease pathology, the phosphorylation of Tau protein is disturbed, leading to hyperphosphorylation and the emergence of new sites of phosphorylation.The combination of decreased concentrations of β-amyloid1-42 and increased concentrations of total Tau and pTau is considered to be a pathological biomarker signature in cerebrospinal fluid (CSF) that is diagnostic for Alzheimer's disease.2,3 Today, CSF biomarker analysis has become part of routine clinical testing, however, measuring biomarkers in blood could improve future test strategies.
Current research indicates that plasma pTau, including pTau 181, is concordant with amyloid PET, and is able to differentiate between Alzheimer's disease and non-Alzheimer's disease neurodegenerative diseases and to predict progression to Alzheimer's disease.4-7 Blood-based biomarkers, such as plasma pTau, could potentially be used as inclusion criteria or to evaluate target engagement and treatment efficacy, and could further advance the development of disease-modifying treatments in the field of Alzheimer's disease and related disorders.8 This assay is designed to measure specifically the phosphorylation in position 181 threonine in human plasma.
See the LUMIPULSE G1200 working in this video:
References:
- Lewczuk P, et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J Biol Psychiatry, 19(4): 244-328; 2018.
- Olsson B, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol, 15(7): 673-684; 2016.
- Hansson O, et al. Advantages and disadvantages of the use of the CSF Amyloid β(Aβ) 42/40 ratio in the diagnosis of Alzheimer's Disease. Alzheimers Res Ther, 11: 34, 2019.
- Palmqvist S, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer's disease. EMBO Mol Med, 11(12): e11170, 2019.
- Janelidze S, et al. Plasma pTau 181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med, 26(3): 379-386, 2020.
- Mielke M, et al. Plasma phospho-tau181 increases with Alzheimer's disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement, 14(8): 989-997, 2018.
- Karikari T, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol, 19(5): 422-433, 2020.
- Teunissen C, et al. Blood-based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol, 21(1): 66-77, 2022.
-
Conditions of sale
To read the end user conditions of sale for this product please visit our Resource center.
-
Citations
The BIOZ badges associated with Fujirebio products include peer-reviewed citations derived from scientific studies using Fujirebio products. Please note that the peer-reviewed citations do not reflect the regulatory status of Fujirebio products. Users should refer to the specific product documentation and any (clinical) claims made therein in order to ensure compliant use. For each country or geographic region, users must verify the related regulatory status of the Fujirebio product.
-
Documentation
Browse regulatory documents for this product
Create a (free!) eServices account and start browsing all product documentation right away.
Get access to this section and more
Create a free eServices account now and instantly access multiple digital resources:
- Product documentation
- Selected scientific posters
- Product leaflets
- How-to videos and more…
-
Insights
Jul 23, 2025Fujirebio introduces its Neuro Expert Toolbox (NExT) at AAIC 2025
Fujirebio is introducing its Neuro Expert Toolbox (NExT) for the first time at AAIC 2025 (Alzheimer's Association International Conference®) in...
Nov 13, 2024Video - Alzheimer's awareness redefined
Follow the journey of the Sullivan family and leading Alzheimer’s Neurologist and Researcher Dr. David Greeley as they introduce and explain these...
Feb 20, 2024Publication - Serum and cerebrospinal fluid neurofilament light chains measured by SIMOA™, Ella™, and Lumipulse™ in multiple sclerosis naïve patients
We would like to draw your attention to a first publication on our Lumipulse® G NfL solution. In this article you will find a method comparison of CSF...
-
Product inquiry
-
Related products