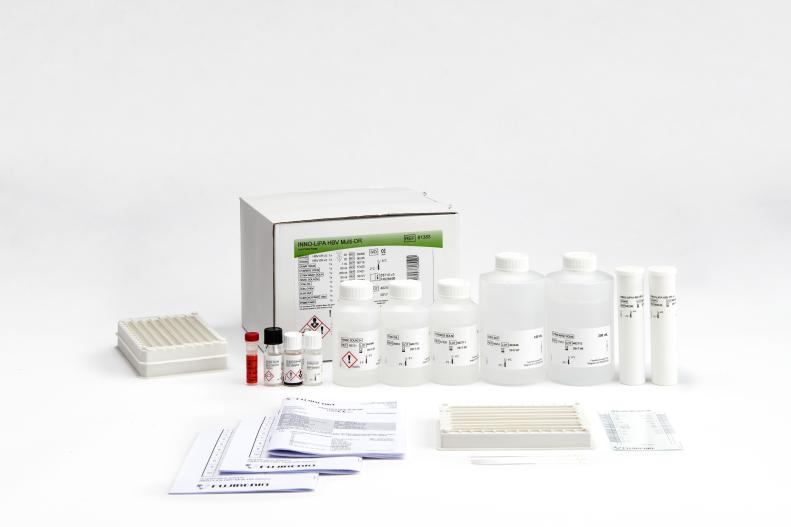
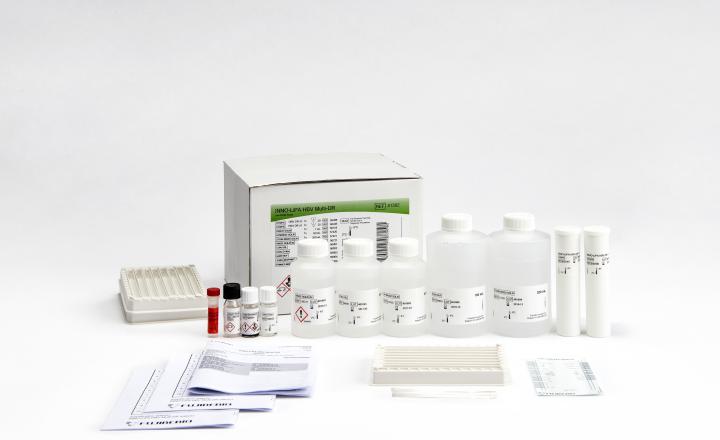
INNO-LIPA® HBV Multi-DR
Minimize treatment failure, Maximize long-term HBV suppression.
Line probe assay for simultaneous detection of hepatitis B virus wild-type motif, lamivudine, telbivudine, emtricitabine, adefovir, and entecavir resistance-associated mutations.
CE marked
INNO-LIPA® HBV Multi-DR
Product number 81383
20 Tests (Amplification kit included)
Please contact your local Fujirebio representative for the availability of this product in your country.
Click here to navigate
- Contact sales for information
- Details
- Conditions of sale
- Citations
- Documentation
- Insights
- Product inquiry
- Related products
-
Details
Features & Benefits
- Earliest detection of mutants:
- Highly sensitive detection at single nucleotide level of mixtures of wild-type and mutants, identifying the appearance of mutants earlier than sequencing and viral load assays
- Lower limit of detection of 990 copies/ml
- Allowing rapid and effective HBV suppression by early adoption to the most suited therapy
- All the necessary information in one assay:
- Allows simultaneous detection of all clinically relevant HBV polymerase wild-type and drug-induced mutations associated with lamivudine, emtricitabine, telbivudine, adefovir and tenofovir resistance as well as known compensatory mutations (codons 80, 173, 180, 181, and 204)
- Identifies amino acid changes in the overlapping reading frame of HBsAg (codons 171, 172, 195, 196, 198, and 199)
- Optimized for 8 known HBV genotypes (A-H)
- Low chance of errors and limited hands-on time:
- Easy to perform and robust assay
- Amplicon can also be used for INNO-LIPA HBV GT
- Fully automated processing of the strips possible with TENDIGO®, Auto-LIPA™ 48 and AutoBlot 3000H.
- Objective and automated reading and interpretation of strips possible using LiRAS® for LiPA HBV.
-
Conditions of sale
To read the end user conditions of sale for this product please visit our Resource center.
-
Citations
-
Documentation
Browse regulatory documents for this product
Create a (free!) eServices account and start browsing all product documentation right away.
Get access to this section and more
Create a free eServices account now and instantly access multiple digital resources:
- Product documentation
- Selected scientific posters
- Product leaflets
- How-to videos and more…
Multimedia
Watch product videos
Get a (free!) eServices account and benefit from full access to all our online resources.
-
Insights
Jul 10, 2019HBcrAg, a promising new serological marker for reliable and non-invasive monitoring of the intrahepatic virologic status in HBV patients
In March 2017 the European Association for the Study of the Liver (EASL) added a new serological marker, HBcrAg (Hepatitis B core-related antigen), to...
-
Product inquiry
-
Related products