INNO-LiPA® CFTRiage
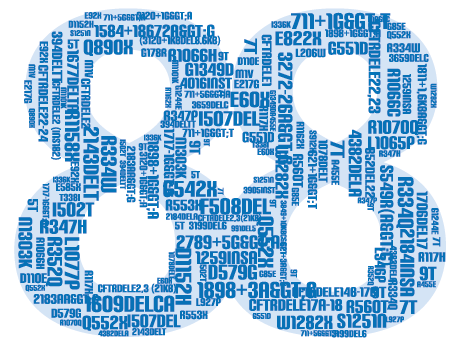
The INNO-LiPA CFTR iage is a line probe assay, intended for the simultaneous in vitro detection and identification of 88 human Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene mutations and their wild type sequence in human whole blood, dried blood spots or buccal brushes.
This qualitative genotyping test provides information for carrier testing in adults of reproductive age, can be used as an aid in newborn screening as well as in confirmatory diagnostic testing.
The assay uses amplification reagents intended for the nucleic acid multiplex amplification of 40 regions of the CFTR gene (regions for some mutations are combined) in only one reaction.
The INNO-LiPA CFTR iage approach provides a multiparameter screening test for CFTR gene mutations and discriminates between healthy normal individuals, healthy carriers, and affected patients.
Product number 80577
Product number 80595
Product number 80596
Product number 80580
Product number 80581
Product number 80578
Product number 80579
Product number 80582