INNO-LIA® HIV I/II Score
The clearest answer in HIV confirmation
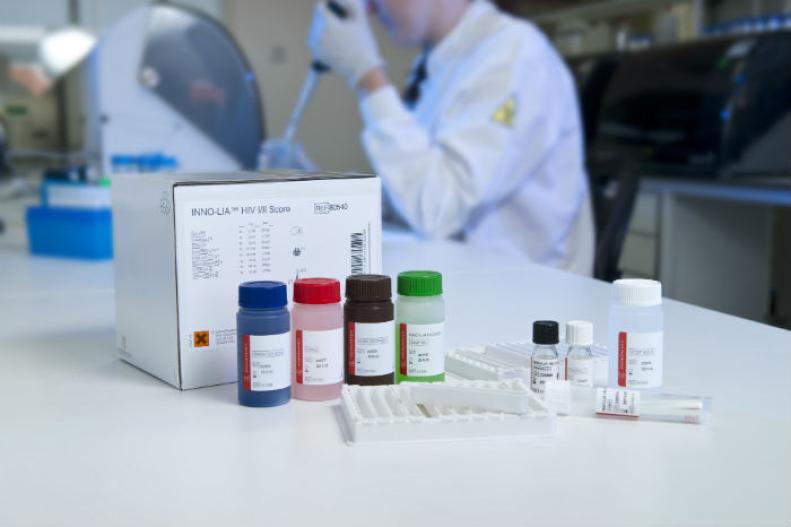
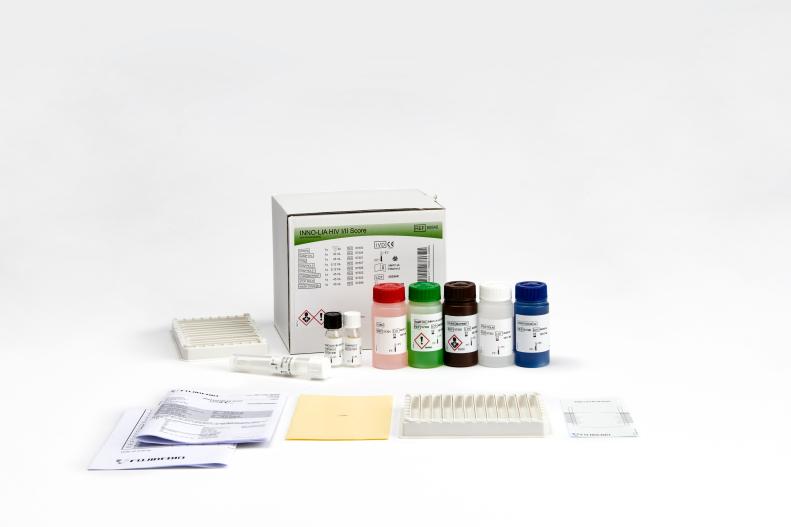
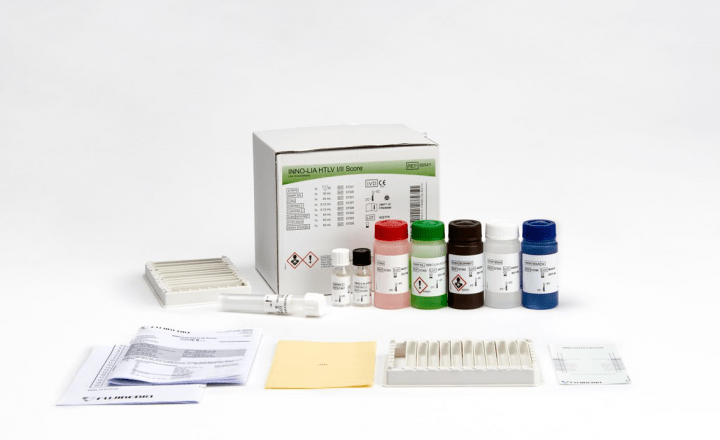
Line immunoassay for the confirmation and discrimination of antibodies to HIV-1, HIV-1 group O and HIV-2 in human serum and plasma.
Product number 80540
Click here to navigate
- Contact sales for information
- Details
- Conditions of sale
- Citations
- Documentation
- Insights
- Product inquiry
- Related products
-
Details
Features & Benefits- All major HIV strains detectable with one strip: HIV-1, HIV-2, and HIV-1 group O
- Optimal specificity in screened-negative blood donors: 96.7% (290/300)
- High sensitivity in BBI panels
- Short and overnight procedures
- Color-coded reagents
- Ready-to-use reagents
- Four control lines to monitor 1) non-specific reactivity, 2) sample addition, 3) and 4) color development steps
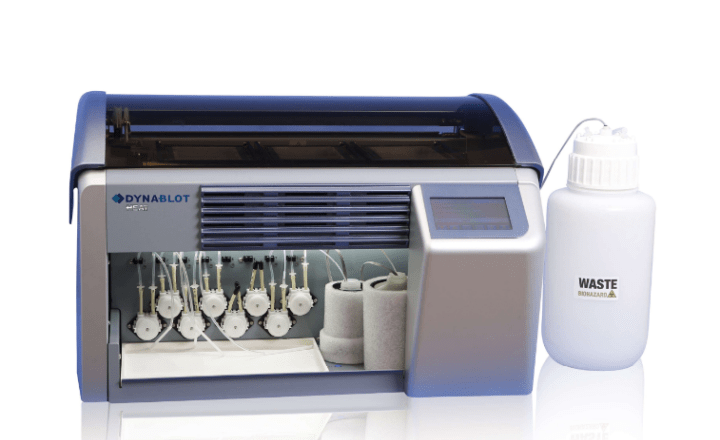
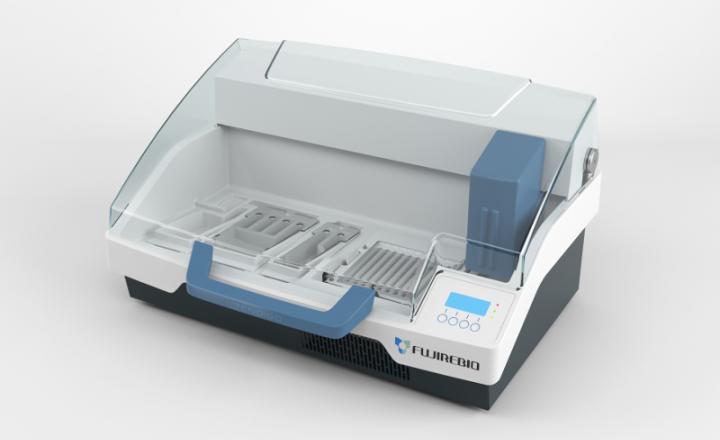
- Fully automated strip processing possible using TENDIGO™, Auto-LIA™ 48, Auto-LiPA™ 48, AutoBlot 3000(H) or Roboblot®
- Objective, automated reading and interpretation of the strips possible using LiRAS® for Infectious Diseases
BackgroundAntibodies to human immunodeficiency virus (HIV) have been detected in most patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex (ARC), and in many asymptomatic individuals belonging to high-risk groups. Enzyme-linked immunosorbent assays (ELISA) are widely used to screen human blood and plasma samples for the presence of these HIV antibodies.
However, since false-positive reactions have frequently been observed with current ELISA tests, it is strongly recommended to confirm repeatedly reactive samples by use of other reliable techniques (such as the INNO-LIA HIV I/II Score). Recombinant proteins and synthetic peptides from HIV-1 and HIV-2, and a synthetic peptide from HIV-1 group O, are coated as discrete lines on a nylon strip with plastic backing.
The INNO-LIA HIV I/II Score assay contains sequences derived from HIV-O sequences to allow the optimal detection of infections caused by this group of viruses. To allow the broad spectrum detection of all HIV infections, the INNO-LIA HIV I/II Score assay also contains separate lines for the differentiation of HIV-1 and HIV-2 infections.
This is important, as HIV-2 is less virulent, resulting in a better prognosis for the patient.
-
Conditions of sale
To read the end user conditions of sale for this product please visit our Resource center.
-
Citations
-
Documentation
Browse regulatory documents for this product
Create a (free!) eServices account and start browsing all product documentation right away.
Get access to this section and more
Create a free eServices account now and instantly access multiple digital resources:
- Product documentation
- Selected scientific posters
- Product leaflets
- How-to videos and more…
Multimedia
Watch product videos
Get a (free!) eServices account and benefit from full access to all our online resources.
-
Insights
May 21, 2019The clearest answer in HIV confirmation testing
When you are confronted with repeatedly reactive HIV screening patient test results it is strongly recommended that you confirm these results with...
-
Product inquiry
-
Related products