PIVKA-II, a biomarker of liver cells for the management of hepatocellular carcinoma (HCC)
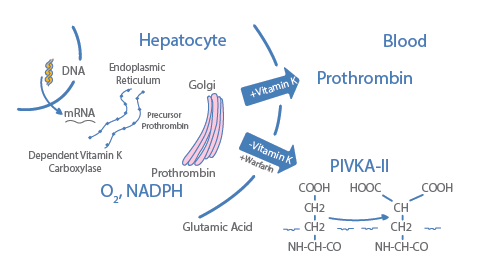
PIKVA-II (Protein Induced Vitamin K Absence or Antagonist-II) is an immature form of prothrombin also known as DCP (Des-gamma-Carboxy Prothrombin), vitamin Kcoagulation factor, synthesized in the liver. In absence of vitamin K or when its action is antagonized (warfarin), PIVKA-II is released into the blood.
Phenotypic changes of liver cells enhance the production of PIVKA-II via impairments of vitamin K uptake1. PIVKA-II is an established HCC marker.
PIVKA-II serum and tissue overexpression are related to worse tumor behavior2-3, such as the presence of (micro) vascular invasion and intrahepatic metastasis of HCC cells and may indicate a poor prognostic outcome.
Clinical background of Hepatocellular carcinoma (HCC)
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide with a high rate of mortality and an increasing incidence. The most common causes of HCC are either a viral hepatitis infection (hepatitis B or C) or cirrhosis (alcoholism being the most common cause of hepatic cirrhosis).
The starting point in the diagnostic surveillance of at risk patients is ultrasound. In a second stage this can be completed with the measurement of a tumor marker called α-fetoprotein (AFP). Since ultrasound has a poor performance in cirrhotic patients and is also operator dependent, further characterization by CT or MRI is often required.
As it is important to detect HCC at an early stage when the disease is still curable, biomarkers can complete the diagnosis obtained with ultrasound before moving on to the more expensive MRI. Biomarkers can also be used to better stratify patients on the liver transplant waiting list. Additionally, the risk of recurrence of HCC after transplantation can be assessed by monitoring patients with biomarkers.
References
- Murata et al. Int J Oncol 2010; 36:161-170
- Inagaki et al. BioScience Trends 2008; 2(2):53-60
- Shirabe et al. J Surg Oncol 2007; 95:235-240
- Tateishi et al. Hepatol Int 2008; 2:17-30
- Hu et al. Int J Mol Sci 2013; 14:23559-580
- Rodriguez-Peralvarez et al. Ann Surg Oncol 2013; 20(1):325-339
- Poté et al. J Hepatol/j.jhep.2014.11.005
- Hakamada et al. World J Gastroenterol 2008; 14(9):1370-77
- Fujiki et al. Am J Transplant 2009; 10:2362-71
- Shindoh et al. Transplant International 2014 ESOT; 27:391–398
- Wang et al. Asian Pac J Cancer Prev 2015; 16(9):3595-3604
- Kondo et al. Clin J Gastroenterol 2015; DOI 10.1007/s12328-015-0568-9
- Marrero et al. HEPATOLOGY 2003; 37(5):1114-21
- Kim et al. Hepato-Gastroenterology 2015; 62:393-394
- Asaoka et al. PLoS ONE 2014; 9(11): e111662