
1950
- Fujirebio (formerly Fujizoki Pharmaceutical, Co., Inc.) is founded in Tokyo, Japan.
1966
- Launch of HA Ag (TPHA), the world’s first hemagglutination test for syphilis.
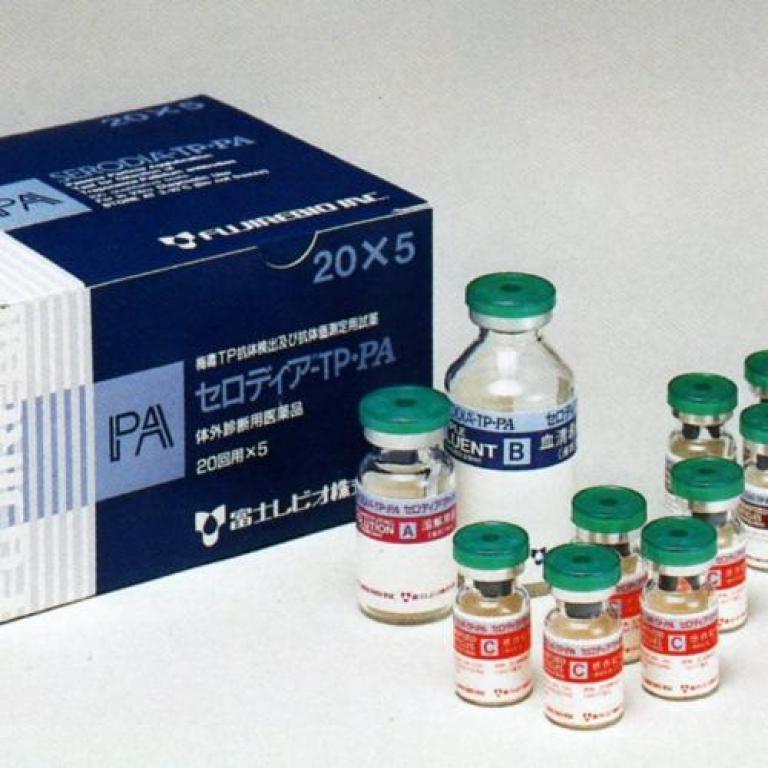
1978
- Launch of the syphilis SERODIA®-TP•PA test kit, destined to become a gold standard.

1981
- Established Fujirebio Taiwan Inc.

1987
- Established Fujirebio America, Inc. (merged to Fujirebio Diagnostics, Inc.) in USA.

1992
- LUMIPULSE® 1200, the world’s first fully automated CLEIA testing instrument, is launched.
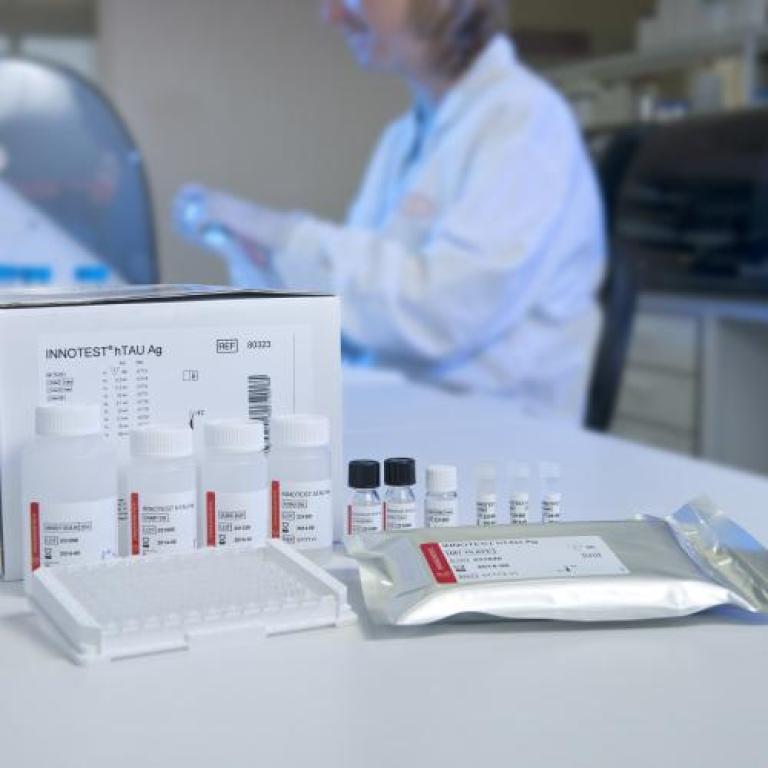
1995
- INNOTEST® hTAU Ag is the world’s first commercialized biomarker kit for early detection of Alzheimer’s disease.

1996
- Launched LUMIPULSE f in Japan.

1998
- Acquisition of Centocor Diagnostics of Pennsylvania, Inc. (USA, currently Fujirebio Diagnostics, Inc.), pioneer in oncology testing and developer of CA125II, CA19-9 and CA15-3.
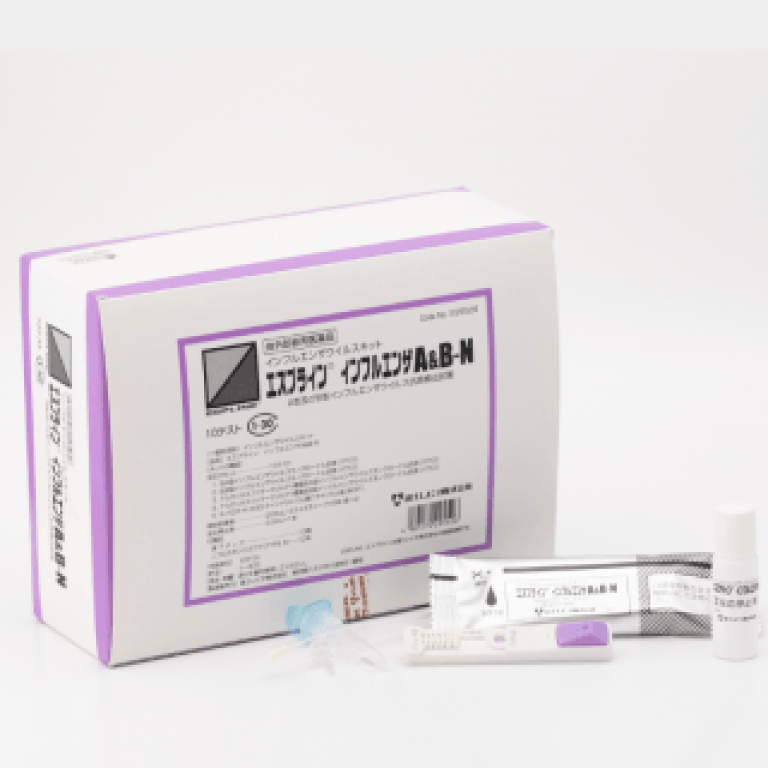
2002
- Launched ESPLINE® flu-A&B.

2005
- Launched LUMIPULSE S and LUMIPULSE Presto II.

2006
- Acquisition of CanAg Diagnostics AB (Sweden, currently Fujirebio Diagnostics AB), leader in oncology biomarker development.

2007
- CL4800 is launched in Japan to support blood screening for Japan Red Cross (~2019).

2008
- Acquisition of Advanced Life Science Institute, Inc.
- Acquisition of American Biological Technologies, Inc. (merged to Fujirebio Diagnostics, Inc.).
- LUMIPULSE G1200, a mid-sized, cartridge-based instrument for fully automated CLEIA testing, is launched.

2010
- Acquisition of Innogenetics N.V. (Belgium, currently Fujirebio Europe N.V.), world leader in specialty molecular and immunoassay testing.

2014
- The LUMIPULSE G600II, a compact cartridge-based CLEIA benchtop analyzer, is launched.

2015
- LUMIPULSE L2400, a high-volume instrument using bottle-type reagents is launched.

2017
- Launched FUXION+ (Immunoassay and Clinical Chemistry platform).
- Established Fujirebio Holdings, Inc., as parent company of Fujirebio Inc., Fujirebio Diagnostics, Inc., Fujirebio Europe N.V. and other Fujirebio group companies.
- Launched Lumipulse G β-Amyloid 1-42 and Lumipulse G Total Tau.
2018
- Launched Lumipulse G β-Amyloid 1-40 and Lumipulse G pTau 181.

2019
- Established Fujirebio Diagnostics Japan, Inc., focusing on OEM business in Japan.
2020
- Launched ESPLINE SARS-CoV-2 and Lumipulse G SARS-CoV-2 Ag.
- Established Fujirebio China Co., Ltd.

2021
- Launched ESPLINE SARS-CoV-2 & Flu A+B.

2022
- Launched Lumipulse G blood-based Alzheimer’s disease biomarker assays for RUO testing of β-amyloid1-42, β-amyloid1-42, and pTau 181.
- Acquisition of ADx NeuroSciences (Belgium), pioneers in generating tailor-made antibodies and developing assays for neurodegenerative diseases.
- Acquisition of Fluxus, Inc. (USA), a Silicon Valley-based innovation company that has a ultra-high sensitivity detection technology in the biotechnology area.

