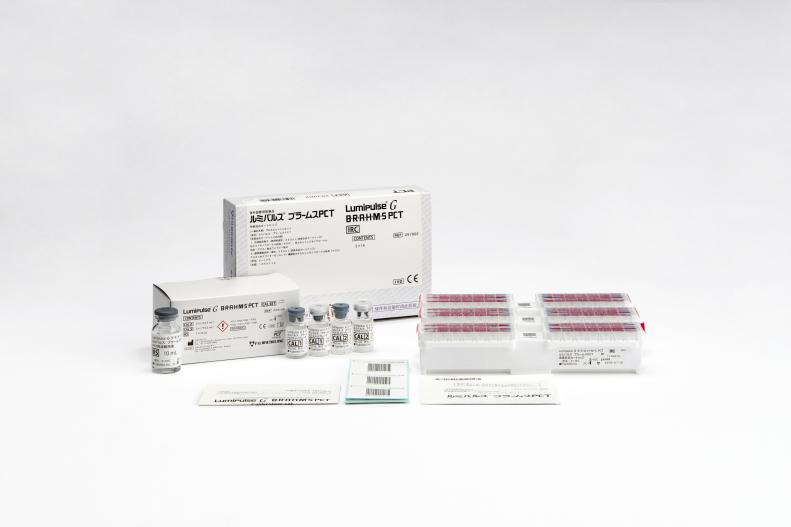
Lumipulse® G B•R•A•H•M•S PCT
The most sensitive fully automated Procalcitonin (PCT) test
Immunoreaction cartridges for in vitro diagnostic (IVD) use with a two-step sandwich immunoassay method on the LUMIPULSE G system for the quantitative determination of procalcitonin (PCT) in human serum and plasma.
The assay utilises proven CLEIA (chemiluminescent enzyme immunoassay) technology with results that are available in up to 35 minutes.
CE marked
Lumipulse G B•R•A•H•M•S PCT Immunoreaction Cartridges
Product number 297902
3 × 14 Tests
Lumipulse G B•R•A•H•M•S PCT Calibrators
Product number 234150
2 × 2 Concentrations
Please contact your local Fujirebio representative for the availability of this product in your country.